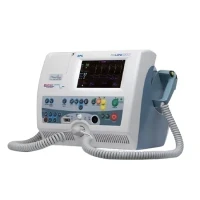
BPL DF2509 Defibrillator Machine
The BPL DF2509 Defibrillator Machine is a medical device used to treat cardiac arrhythmias, particularly in emergencies such as cardiac arrest. It provides electrical shocks to the heart to restore a normal rhythm and is a crucial tool in pre-hospital and hospital settings.
Key Features:
1. Defibrillation Technology:
- Energy Levels: Offers adjustable energy levels for defibrillation, allowing for appropriate shock delivery based on the patient's needs.
- Automated and Manual Modes: Includes both automated and manual defibrillation options. Automated mode guides shock delivery, while manual mode allows healthcare providers to adjust settings manually.
2. Monitoring Capabilities:
- ECG Monitoring: Equipped with ECG monitoring capabilities to visualise the heart’s electrical activity and assist in diagnosing the arrhythmia.
- Display: Features a clear digital display for real-time monitoring of the patient’s cardiac rhythm and other vital signs.
3. User Interface:
- Intuitive Controls: Designed with user-friendly controls and interfaces for easy operation during critical situations.
- Touchscreen or Button Controls: Depending on the model, may include a touchscreen or physical buttons for operation.
4. Safety Features:
- Patient Safety: Includes safety mechanisms to prevent inappropriate shocks and ensure proper electrode placement.
- Battery Backup: Often comes with a battery backup to ensure functionality during power outages or in mobile settings.
5. Connectivity and Data Management:
- Data Storage: Capable of storing patient data and defibrillation events for review and analysis.
- Data Export: May offer options to export data for integration with electronic health records (EHR) or for further documentation.
6. Portability and Design:
- Compact and Lightweight: Designed to be portable and easy to carry, making it suitable for emergencies in various environments.
- Durable Construction: Built to withstand the rigours of emergency medical use.
7. Training and Simulation:
- Training Modes: Some models include training modes or simulations to help users practice defibrillation techniques and familiarize themselves with the device.
8. Compliance and Certification:
- Regulatory Compliance: Meets relevant medical device standards and regulations to ensure safety, effectiveness, and reliability.
Applications:
- Emergency Medical Services (EMS): Used by paramedics and emergency medical technicians (EMTs) in pre-hospital settings to treat cardiac arrest.
- Hospitals: Essential in emergency departments, intensive care units (ICUs), and other hospital areas for managing acute cardiac events.
- Public Access: May be placed in public areas, such as airports or schools, as part of an Automated External Defibrillator (AED) program.
Benefits:
- Critical Life-Saving: Provides essential support for cardiac patients in life-threatening situations, improving chances of survival.
- Ease of Use: Designed to be intuitive and accessible for healthcare providers with varying levels of experience.
- Versatility: Suitable for use in a wide range of medical settings, from emergency response to hospital care.
